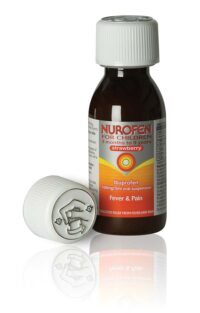
 United Closures and Plastics of Norwich is completing an investment in its PP28mm Medi-Loc® capacity to meet increased demand anticipated from a recent MHRA voluntary code to extend the use of child resistant packaging to BS EN ISO 8317 2004 for all liquid formulations available over-the-counter authorised for the treatment of coughs and colds primarily for the children’s market.
United Closures and Plastics of Norwich is completing an investment in its PP28mm Medi-Loc® capacity to meet increased demand anticipated from a recent MHRA voluntary code to extend the use of child resistant packaging to BS EN ISO 8317 2004 for all liquid formulations available over-the-counter authorised for the treatment of coughs and colds primarily for the children’s market.
The voluntary code comes into practice in September 2010 for all relevant products containing the ingredients reviewed (including adult formulations) coming into the supply chain. This move follows regulations, which came into force in October 2005 requiring the use of child resistant packaging for liquid pharmaceutical products containing paracetamol, aspirin and specified vitamins.
UCP’s PP28mm Medi-Loc® is the leading child resistant closure in the UK liquid pharmaceuticals market and, in recognition of the steadily increasing age of our population, has also been tested in the 60 – 80 years age range, achieving a 100% pass rate. UCP’s investment allows for the resultant growth in expected demand for child resistant closures as a result of this voluntary code.
United Closures & Plastics Ltd
Tel UCP Norwich: 01603 894800




Comments are closed.